TTP Research
Thrombosis Research: In patients with suspected immune TTP, admission source impacts hospital length of stay and time to therapeutic plasma exchange impacts clinical outcomes
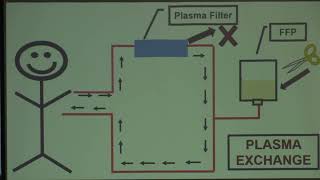
When immune thrombotic thrombocytopenic purpura (TTP) is suspected, outcomes are impacted by time to therapeutic plasma exchange (TPE). We evaluated the impact of time to TPE on outcomes in suspected TTP cases admitted through the Emergency Department (ED) vs. transferred from another facility (Transfer). Read the article here.
British Journal of Haematology: Paediatric patients with suspected immune thrombotic thrombocytopenic purpura also experience treatment delays
The Goal of this article is to Assess treatment delays in paediatric patients with TTP. Read the article.
Journal of Clinical Medicine: Patient-Reported Outcome Measures in Patients with Thrombotic Thrombocytopenic Purpura: A Systematic Review of the Literature
This goal of this article is to define the landscape of PROMs at present and their validity evidence in studies of patients with TTP. Read the article.
Blood Advances: “Health Following Recovery from Immune Thrombotic Thrombocytopenic Purpura: The Patient’s Perspective”, Blood Advances
Blood Adv 2023; 7 (9): 1813–1822. doi: https://doi.org/10.1182/bloodadvances.2022008342
Authors: Rachel A. Kelley, Marshall K. Cheney, Clare M. Martin, Spero Cataland, Lauren B. Quick, San Keller, Sara K. Vesely, Amanda J. Llaneza, Mohamad O. Khawandanah, Janna M. Journeycake, Julie A. Panepinto, Deirdra R. Terrell
PRESS RELEASE: Takeda’s ADZYNMA (ADAMTS13, recombinant-krhn) Approved by U.S. FDA as the First and Only Recombinant ADAMTS13 Enzyme Replacement Therapy for the Treatment of Congenital Thrombotic Thrombocytopenic Purpura (cTTP)
OSAKA, Japan, and CAMBRIDGE, Massachusetts, November 9, 2023 – Takeda (TSE:4502/NYSE:TAK) today announced that the U.S. Food and Drug Administration (FDA) has approved ADZYNMA (ADAMTS13, recombinant-krhn) for the prophylactic and on-demand treatment of adult and pediatric patients with congenital thrombotic thrombocytopenic purpura (cTTP). ADZYNMA is the first and only FDA-approved recombinant ADAMTS13 (rADAMTS13) protein designed to address an unmet medical need in people with cTTP by replacing the deficient ADAMTS13 enzyme. Read more
PRESS RELEASE: Pivotal Phase 3 Data Presented at ISTH 2023 Congress Spotlight TAK-755 Prophylaxis for Patients with Congenital Thrombotic Thrombocytopenic Purpura (cTTP)
On Sunday, June 25, 2023, Takeda announced, interim data from our pivotal, Phase 3 clinical trial on TAK-755 (recombinant ADAMTS13) replacement therapy for the prophylactic treatment of congenital thrombotic thrombocytopenic purpura (cTTP). Read more.
Clinical Trials, Studies, and TTP Support
A Pilot Study of Efgartigimod for Immune-mediated Thrombotic Thrombocytopenic Purpura (iTTP)
Official ID: NCT06831058
Start Date: May 1, 2025 – Ongoing until May 2028, with results expected by May 2029
Sponsor: University of Minnesota
Why it matters:
- iTTP is a rare autoimmune disorder where patients experience dangerous drops in platelet count and risk life-threatening relapses.
- This study explores whether efgartigimod—an FcRn antagonist—can temporarily lower harmful IgG antibodies that contribute to the disease
- The goal: boost ADAMTS13 activity levels to help prevent relapse and stroke during treatment.
Clinical Trials
Have questions? We’ve got answers. Read more.
ADZYNMA Patient Support
cTTP is very rare and difficult to treat. ADZYNMA offers relief for a number of patients. If you need help obtaining medication for cTTP click the link below for patient assistance. Read more.
MAYARI Study
Living with iTTP? Worried about relapse? If you have an acute episode the MAYARI Study may interest you. Learn more.
Thrombocytopenia Copay Assistance Program (The Assistance Fund)
TAF is currently accepting requests to join the enrollment waitlist for this program. Waitlists are administered on a calendar-year basis. At the end of each calendar year, waitlist applications expire. Patients still seeking assistance must join waitlists for the subsequent calendar year. Learn more.
Cablivi Patient Support
Help your aTTP/iTTP* patients transition from hospital to home with CABLIVI. Read more.
Patient Highlights
Charlotte Magazine, “A Rare Disorder That Emerged From Nowhere Nearly Killed This Lake Wylie Man”
Rob Marchand was a healthy mathematician from Lake Wylie—until his world flipped upside down. Out of nowhere, he collapsed and was diagnosed with TTP (thrombotic thrombocytopenic purpura)—a rare, life-threatening blood disorder.
After plasma exchanges, hospital stays, and a long road to recovery, Rob’s story is a powerful reminder that rare diseases don’t always come with warning signs.
Learn about TTP, Rob’s fight to survive, and how this medical mystery changed his life. Read his story.
Black Health Matters.com, “You May Have This Disorder—And Not Even Know It”
Black Health Matters highlighted our Beloved Reeshemah Wynn.
Reeshemah Wynn went to the emergency room with what she thought was the flu, but she was later diagnosed with a rare blood disorder called aTTP. Read her story.
Additional Patient Resources
Understanding TTP
What you need to know about thrombotic thrombocytopenic purpura (TTP). TTP is a serious condition that can be managed when treated quickly. Learn more.
Patients and Families Overview
USTMA Patient Resources
Learn more about USTMA’s free patient resources.
USTMA Physician Resources
Learn more about USTMA’s free physician resources.
Orpha.net, Portal for Rare Diseases
Learn more about Thrombotic thrombocytopenic purpura at Oprha.net, The portal for rare diseases and orphan drugs.
Videos